Background
Patients with 2017 European LeukemiaNet (ELN) adverse risk acute myeloid leukemia (AML) are at 40% risk of relapse leading to poor survival after allogeneic hematopoietic cell transplantation (allo-HCT) (Hansen TCT 2021, Jimenez BMT 2021). Ex vivo donor-derived gamma delta T cells (GDT) expanded with artificial antigen presented calls (aAPC) are a novel therapy with potent MHC-independent antineoplastic cytotoxicity. An ongoing phase 1/1b study is evaluating the safety and potential efficacy of ex vivo expanded donor GDT after reduced-intensity conditioning (RIC) allo-HCT in patients with ELN adverse risk AML ( NCT05015426). We report on manufacturing, safety, and correlative biology of expanded donor GDT after allo-HCT in the first 5 treated patients (Table 1).
Methods
The primary endpoint is the maximum-tolerated dose (MTD) of allogeneic donor GDT as a single infusion at day 60 after RIC allo-HCT. Next generation sequencing was used for measurable residual disease (MRD) assessment pre-HCT and post-GDT infusion. Donor GDT collected from the same donor of the allo-HCT were enriched from non-mobilized leukapheresis product by stimulation with zoledronic acid and IL-2 for 7 days followed by alpha beta T cell depletion using the Miltenyi CliniMACS. Enriched GDT (>90% TCRVδ2) were then co-cultured with irradiated aAPCs in Wilson Wolf G-Rex 100MCS over 10 days (Boucher J. Immunother 2023). This single center, investigator-initiated phase 1 trial is testing 3 distinct dose cohorts of infused GDT (5 x 10 6 cells/kg, 2.5 x 10 7 cells/kg, and 1 x 10 8 cells/kg) with acceptable range of 25% margin. A Bayesian optimal interval design is used to guide dose escalation/de-escalation decisions.
Results
First 3 patients enrolled in dose level 1 (DL1) cohort received GDT dose of 6.25 x 10 6 cells/kg, while last 2 patients enrolled in DL2 cohort received 3.13 x 10 7 cells/kg dose. Dose-limiting toxicities (DLT) were evaluated for 42 days following GDT infusion and no DLT were observed. None of the treated patients experienced cytokine release syndrome (CRS), Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) or graft-versus-host disease (GVHD) during DLT period. Among patients treated on DL1, patient #01 had ASXL1 AML with MRD pos (VAF 43.9%) prior to RIC haploidentical (Haplo) HCT. He is in ongoing complete remission (CR) 9 months post-HCT and has had no GVHD. Patient #02 had MRD pos (VAF 61.7%) AML with TP53 mutation/complex karyotype prior to RIC matched sibling donor allo-HCT. He is in CR and has mild chronic GVHD at 7 months post-HCT. Patient #03 with pre-HCT MRD negRUNX1 AML is in ongoing CR 7 months post-RIC Haplo HCT with no GVHD. At day 28 and day 120 post-GDT, all 3 patients were MRD neg in bone marrow and had 100% bone marrow and peripheral blood leukocyte and granulocyte chimerism. In DL2 cohort, patient #04 with RUNX1 AML and patient #05 with t(9;22) AML both were MRD neg prior to RIC Haplo HCT and remain in CR with no GVHD at 5 and 4 months post-HCT, respectively. At day 28 post-GDT, both patients were MRD neg and had 100% chimerism. Patient #05 had a fall during DLT period in a setting of orthostatic hypotension requiring hospitalization for 24 hours for hydration. This was the only grade 3 adverse event observed in this trial.
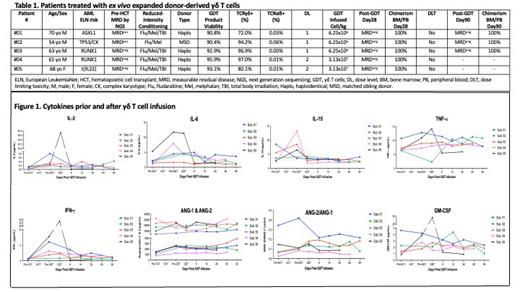
Serum cytokines analyzed included IL2, IL6, IL15, IFNγ, TNFα, Angiopoietin 1&2, and GM-CSF (Figure 1). We compared results prior and up to 90 days post-GDT infusion and found no significant differences, which can explain no CRS or ICANS events yet observed on this trial. Immunophenotype (TCRVδ2, TCRVδ1, PD1, CTLA4, TIGIT and CD28) of GDT was analyzed in samples of donor apheresis, pre- and post-enrichment, infused GDT product and at days 28 and 90 post-infusion. GDT (TCRVδ2) persisted at day 28 and day 90 post-infusion and expressed low levels of cell surface inhibitory receptors.
Conclusion
This preliminary interim report demonstrates favorable safety and tolerability of donor GDT therapy with no CRS, ICANS or GVHD in first 5 patients treated in this trial. All these patients with adverse risk AML are in ongoing MRD neg CR following RIC allo-HCT, including 2 pre-HCT MRD pos cases with TP53 AML and AXSL1 AML. Early results of this trial are promising, which provides validation for continued testing of adoptive transfer of ex vivo expanded donor GDT. Updated results of this trial will be presented at the annual conference.
Disclosures
Bejanyan:Magenta Therapeutics: Consultancy; Sanofi: Consultancy; AlloVir: Consultancy; CTI BioPharma: Consultancy; CareDx Pharma: Consultancy; Orca Bio: Consultancy; Medexus Pharmaceuticals: Consultancy; CRISPR Therapeutics: Research Funding. Elmariah:Bristol Myers Squibb: Research Funding. Faramand:Gilead: Research Funding; Kite: Research Funding. Liu:BioLineRx: Membership on an entity's Board of Directors or advisory committees. Sallman:Aprea, Jazz: Research Funding; AbbVie, Affimed Gmbh, Gilead, Incyte, Intellisphere, LLC, Molecular Partners AG, PGEN Therapeutics, Inc., Takeda, Zentalis; Advisory board for AvenCell, BlueBird Bio, BMS, Intellia, Jasper Therapeutics, Kite, Magenta Therapeutics, NKARTA, Novartis, Orbita: Consultancy. Lancet:Atheneum: Consultancy; AbbVie Inc.: Consultancy; BerGenBio / DAVA Oncology: Consultancy; Boxer Capital: Consultancy; Celgene: Consultancy, Research Funding; Globe Life Sciences: Consultancy; Jasper Therapeutics: Consultancy; Jazz: Consultancy; MD Anderson: Consultancy; MEDTalks: Consultancy; Novartis: Consultancy; Peer Voice: Consultancy; Servier: Consultancy; Tegus: Consultancy; The Dedham Group: Consultancy. Locke:Cellular Medicine Group: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imedex: Other; Aptitude Health: Other: Travel Support; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional ; Calibr: Consultancy; Caribou: Consultancy; CERo Therapeutics: Other: (Institutional); ASH: Other: Travel Support; Kite, a Gilead Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; National Cancer Institute: Other; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional; Iovance: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sana: Consultancy, Membership on an entity's Board of Directors or advisory committees; Society for Immunotherapy of Cancer: Other; Clinical Care Options Oncology: Other; Bristol Myers Squibb/ Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional , Research Funding; Cowen: Consultancy; Umoja: Consultancy, Membership on an entity's Board of Directors or advisory committees; EcoR1: Consultancy; Daiichi Sankyo: Consultancy; BioPharma Communications CARE Education: Other: Institutional; Leukemia and Lymphoma Society: Other; Wugen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Emerging Therapy Solutions: Consultancy, Other; Gerson Lehrman Group (GLG): Consultancy; GammaDelta Therapeutics: Consultancy; A2 Biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Davila:Atara Biotherapeutics: Consultancy; Adaptive Biotechnologies: Other: Ownership interest (stock, stock options in a publicly owned company); CRISPR (CRSP): Patents & Royalties: Intellectual property rights (Royalties or patent sales); Synthekine: Consultancy; Syncopation Life Sciences: Consultancy; Precision Biosciences: Other: Ownership interest (stock, stock options in a publicly owned company); Legend Biotech: Consultancy; Kite Pharma Inc.: Other: Teaching and Speaking; Caribou Biosciences: Consultancy; Capstan: Other: Advisor or review panel participant; Bellicum Pharmaceuticals, Inc.: Other: Advisor or review panel participant; Ownership interest (stock, stock options in a publicly owned company); Adicet: Consultancy.